Analysis on the Management System and Policy of China's Pharmaceutical Manufacturing Industry in 2018
1. Regulatory characteristics of the pharmaceutical industry
The state's regulation of the pharmaceutical industry is mainly in four aspects:
(1) Industry license
The state implements an industry entry permit system for pharmaceutical production and operation enterprises, and a pharmaceutical production enterprise or a drug management enterprise must obtain a Pharmaceutical Production License or a Pharmaceutical Business License from the drug regulatory authority.
(2) Mandatory quality management
Implemented mandatory quality management certification (GMP, GSP certification) for the production and operation of pharmaceuticals.
(3) Product license
Registration management is carried out for the production of pharmaceuticals, and the pharmaceutical manufacturer must obtain the drug approval number before it can be produced. Otherwise, the drug cannot be marketed.
(4) National standards
Drugs produced by pharmaceutical manufacturers must implement drug registration standards and must not be lower than the Chinese Pharmacopoeia drug standards promulgated by the drug regulatory authorities of the State Council.
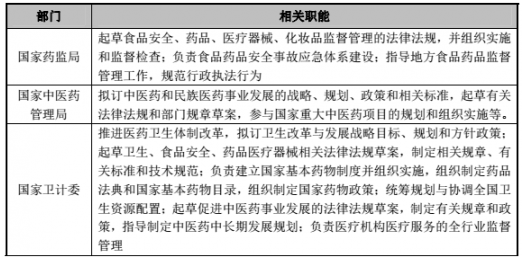
2. Industry authorities
The main departments involved in the supervision system of China's pharmaceutical industry include the State Food and Drug Administration and its local agencies, the State Administration of Traditional Chinese Medicine and its local institutions at all levels, the State Health Planning Commission, and the National Development and Reform Commission.

3. Industry regulations and policies
(1) Major laws and regulations in the pharmaceutical industry
Reference to the research and release world "2018 China pharmaceutical manufacturing industry analysis report - market operation situation and investment prospects forecast"
The laws and regulations related to China's pharmaceutical industry mainly include: "Drug Administration Law of the People's Republic of China", "Regulations on the Implementation of the Drug Administration Law of the People's Republic of China", "Chinese Medicine Law of the People's Republic of China", "Measures for the Supervision and Administration of Pharmaceutical Production", and "Pharmaceutical Management" Measures for the Administration of Licenses, Measures for the Administration of Drug Registration, Measures for the Supervision and Administration of Drug Circulation, Measures for the Administration of Drug Recalls, Measures for the Administration of the Classification of Prescription Drugs and Non-Prescription Drugs (Trial), Drugs Packaging Materials and Container Management Measures ( "Interim)", "Policy Government Pricing Measures", "Pharmaceutical Production Quality Management Regulations (2010 Revision)", "Pharmaceutical Management Quality Management Regulations (2016 Revision)", "New Drug Registration Special Approval Management Regulations", "Drug Instructions and Regulations on the Use of Labels, the Code of Practice for Drug Prices, the Implementation Opinions on Establishing a National Essential Drug System, and the National Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug List.
(2) Major policies and plans for the pharmaceutical industry
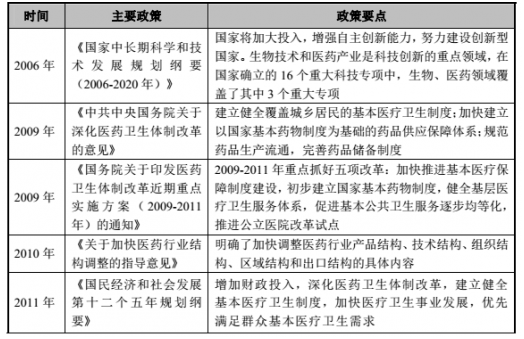
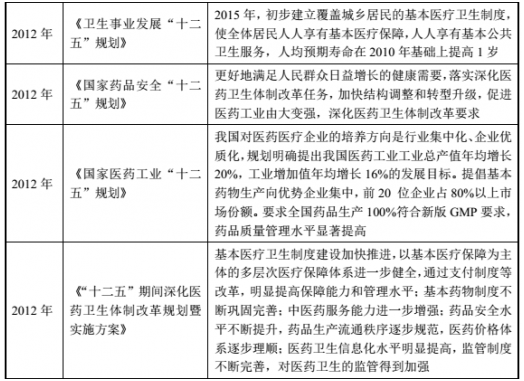
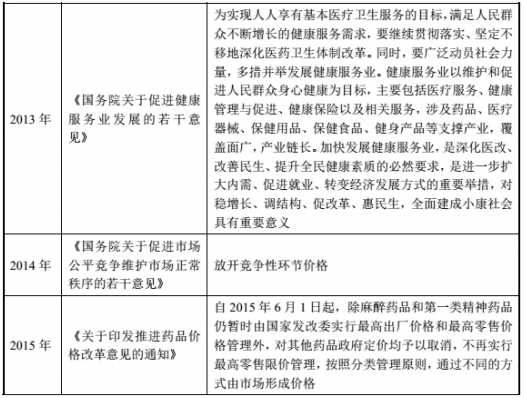
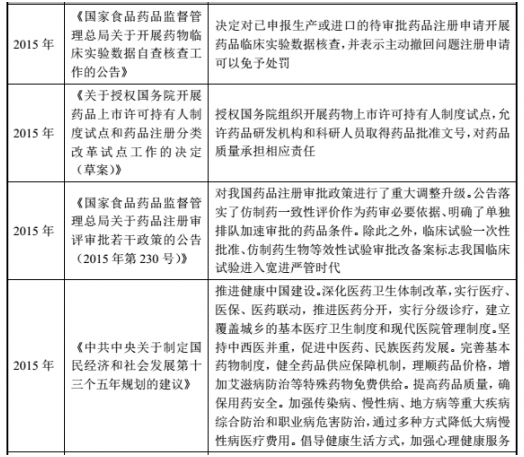

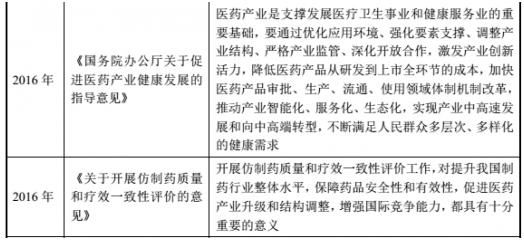

(3) Policy and development planning of the Chinese medicine industry
The Chinese medicine industry is an area that China has always attached great importance to and focused on. In recent years, the state has successively issued the "Outline of Chinese Medicine Innovation and Development Plan", "Several Opinions on Supporting and Promoting the Development of Traditional Chinese Medicine", and "13th Five-Year Plan for the Development of Traditional Chinese Medicine", etc., supported by industrial policies and basic Further policy support was provided in various aspects such as the construction of the drug system and the development of the Chinese medicine industry. In the Outline of the National Medium- and Long-Term Science and Technology Development Plan (2006-2020), the state explicitly requires the Chinese medicine industry to carry out theoretical innovation and research to promote the stable and healthy development of the industry.
In 2015, the General Office of the State Council issued the “Chinese Medicine Health Service Development Plan (2015-2020)”, which mentioned that by 2020, the Chinese medicine health service system will be basically established, and the Chinese medicine health service will accelerate development and become China's health. The important strength of the service industry and the important manifestation of international competitiveness have become an important force in promoting economic and social transformation and development.
In 2016, the State Council issued the Outline of the Strategic Plan for the Development of Traditional Chinese Medicine (2016-2030). The document mentioned that by 2030, the modernization level of TCM governance system and governance capacity will be significantly improved, and the coverage of TCM services will be fully covered. The ability of Chinese medicine health services has been significantly enhanced, the leading role in the treatment of disease, the synergy in the treatment of major diseases, and the core role in the rehabilitation of diseases have been fully exerted; the level of science and technology of Chinese medicine has improved significantly, and it has basically formed a hundred The team of Chinese medicine professionals consisting of masters of Chinese medicine, 10,000 famous Chinese medicine practitioners, millions of Chinese medicine practitioners, and thousands of vocational and technical personnel; the cultural quality of Chinese medicine practitioners has been greatly improved; the level of intelligentization of Chinese medicine industry has reached a new level, for economic and social development. The contribution rate has been further enhanced. China's leading position in the development of traditional medicine in the world has been further consolidated. The realization of traditional Chinese medicine inherits innovation and development, coordinated development, ecological green development, inclusive and open development, and people's shared development, laying a solid foundation for healthy China's construction.
Source: Guanyan World








